

Dermata Therapeutics Transforming topical treatment of the skin Corporate Presentation July 2021 Filed pursuant to Rule 433 of the Securities Act of 1933 Issuer Free Writing Prospectus dated July 12, 2021 Relating to the Preliminary Prospectus dated June 10, 2021 Registration Statement No. 333-256997

We have filed a registration statement on Form S-1 (including a preliminary prospectus) with the SEC for the offering to which this presentation relates. The registration statement has not yet become effective. Before you invest, you should read the preliminary prospectus in the registration statement (including the risk factors described therein) and other documents we have filed with the SEC for more complete information about our company and the offering. You may get these documents for free by visiting EDGAR on the SEC web site at http://www.sec.gov/. The preliminary prospectus is available on the SEC website at http://www.sec.gov. When available, electronic copies of the preliminary prospectus supplement and the accompanying prospectus may also be obtained from the offices of Maxim Group LLC, Prospectus Department, 300 Park Avenue., New York, NY, 10022; Telephone: (800) 724-0761; Email: syndicates@maximgrp.com. CAUTIONARY NOTE ON FORWARD LOOKING STATEMENTS AND DISCLAIMERS PROPRIETARY INFORMATION FREE WRITING PROSPECTUS This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our current and future financial performance, business plans and objectives, current and future clinical and preclinical development activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trials and related data, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, competitive position, industry environment and potential market opportunities. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” and similar expressions are intended to identify forward looking statements. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors including, but not limited to, those related to the success, cost and timing of our product candidate development activities and ongoing and planned clinical trials; our plans to develop and commercialize targeted therapeutics, including our lead product candidates DMT310 and DMT410; the progress of patient enrollment and dosing in our clinical trials; the ability of our product candidates to achieve applicable endpoints in the clinical trials; the safety profile of our product candidates; the potential for data from our clinical trials to support a marketing application, as well as the timing of these events; our ability to obtain funding for our operations, development and commercialization of our product candidates; the timing of and our ability to obtain and maintain regulatory approvals; the rate and degree of market acceptance and clinical utility of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; our commercialization, marketing and manufacturing capabilities and strategy; future agreements with third parties in connection with the commercialization of our product candidates; our expectations regarding our ability to obtain and maintain intellectual property protection; our dependence on third party manufacturers; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our ability to identify additional product candidates with significant commercial potential consistent with our commercial objectives; and our estimates regarding expenses, future revenue, capital requirements and needs for additional financing. We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. Moreover, we operate in a very competitive and rapidly changing environment, and new risks may emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed herein may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. We undertake no obligation to publicly update any forward-looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. This document contains proprietary information that is the property of the company. Neither this document, nor the proprietary information contained herein, shall be published, reproduced, copied, disclosed or used for any other purpose, other than the review and consideration of this document.
Corporate Highlights Unique, multi-use topical platform technology utilizing multiple mechanisms of actions Lead program with compelling Phase 2b clinical data for once-weekly topical treatment of acne Pipeline addressing large medical and aesthetic dermatology market opportunities Multiple clinical milestones anticipated in 2021 and 2022 Experienced management team and board DMT310 DMT410 Psoriasis: Phase 1b PoC Initiated – Q1’21 Potentially inhibits inflammatory cytokines IL-17A and IL-17F Anticipated once-weekly topical application Rosacea: Phase 2 Planned – H2’21 Similar inflammatory lesions to acne Reduces IL-17, which facilitates neutrophil recruitment Acne: Phase 3 Planned – H2’22 Potential anti-inflammatory effects Reduced lipogenesis of sebocytes in-vitro Hyperhidrosis: Phase 1b PoC Completed Topical delivery of botulinum toxin Reduction in sweat production Aesthetics: Phase 1b PoC Ongoing - Results Q3’21 Potentially broadens uses for botulinum toxin with topical applications Potentially decreases need for injections *PoC: Proof of Concept

Gerry Proehl – Founder, Chairman and President & CEO Former Founder, President & CEO, Santarus prior to sale to Salix Pharmaceuticals for $2.6 billion, VP, Global Marketing, Hoechst Marion Roussel (now part of Sanofi) Tom Insley – Chief Financial Officer Former CFO, SkinMedica; Former Managing Partner, PwC Chris Nardo, M.P.H., Ph.D. – SVP, Development Former Senior Director, Clinical Development, Allergan; Former VP, Clinical Operations, Spectrum Pharmaceuticals Maria Bedoya Toro Munera, Ph.D. – SVP, Regulatory Affairs & Quality Assurance Former SVP, Regulatory Affairs & Quality Assurance, Receptos and Santarus Experienced Management Team

Gerry Proehl – Founder, Chairman and President & CEO Former Founder, President & CEO, Santarus prior to sale to Salix Pharmaceuticals for $2.6 billion; Former VP, Global Marketing, Hoechst Marion Roussel (now part of Sanofi) David Hale – Founder and Lead Director Chairman & CEO, Hale Bioventures; Former Chairman, Santarus; Former Chairman, SkinMedica prior to sale to Allergan; Former Chairman, Former Chairman of Micromet prior to sale to Amgen Wendell Wierenga, Ph.D. – Director Former EVP, R&D, Santarus; Former EVP, R&D, Ambit Biosciences and Neurocrine Biosciences; Former CEO, Syrrx (acquired by Takeda in 2005); Board of Directors, Cytokinetics and SRI International Kathleen Scott – Director* Current CFO of Neurana Pharmaceuticals; Former CFO of Recros Medica; Former partner at RA Capital Advisors Steven Mento, Ph.D. – Director* Current director of Histogen; Former CEO of Conatus Pharmaceuticals; Former CEO of Idun Pharmaceuticals Mary Fisher – Director* Current CEO and Chair at Colorscience; Former COO of Acorda Therapeutics; Current director of Sientra; Former director of ZELTIQ Aesthetics Andrew Sandler, M.D. – Director* Current CMO at Kiadis Pharma; Former SVP, Medical Affairs of Medivation Experienced Board *Will be elected to the board of directors effective immediately upon the effectiveness of IPO
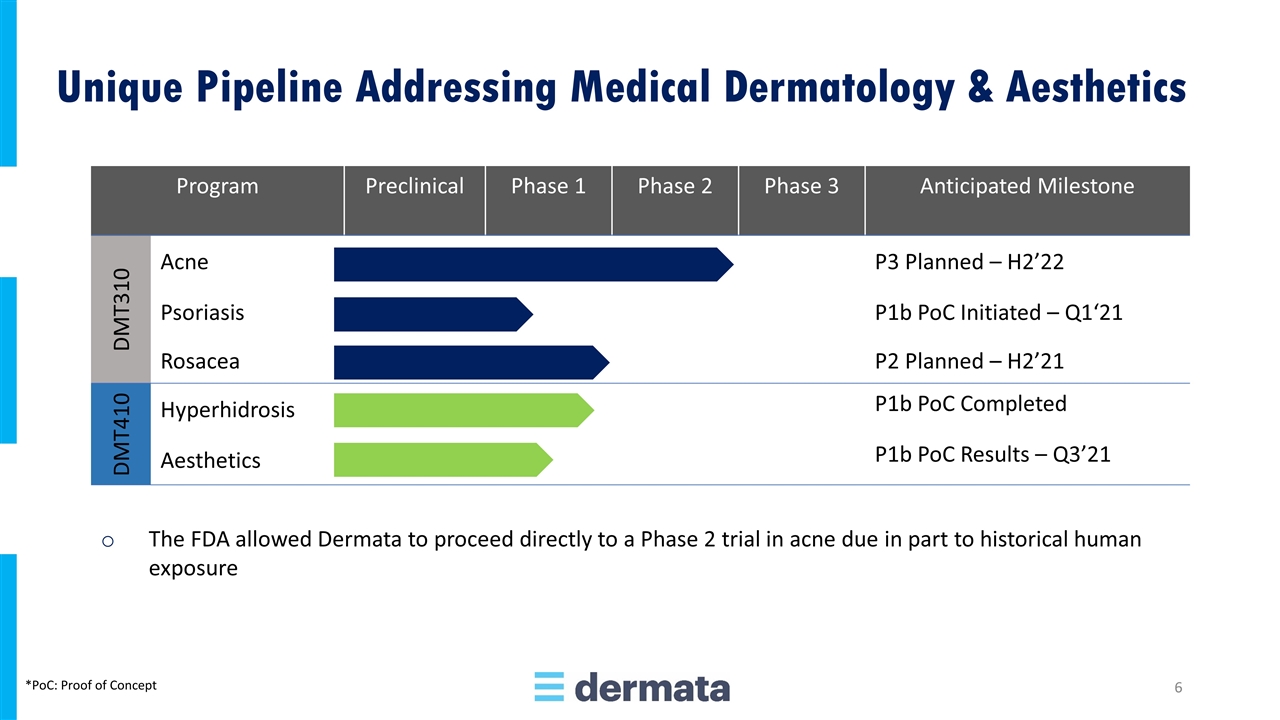
Program Preclinical Phase 1 Phase 2 Phase 3 Anticipated Milestone DMT310 Acne P3 Planned – H2’22 Psoriasis P1b PoC Initiated – Q1‘21 Rosacea P2 Planned – H2’21 DMT410 Hyperhidrosis P1b PoC Completed Aesthetics P1b PoC Results – Q3’21 The FDA allowed Dermata to proceed directly to a Phase 2 trial in acne due in part to historical human exposure Unique Pipeline Addressing Medical Dermatology & Aesthetics *PoC: Proof of Concept
Acne 50 million US patients have acne, with about 85% of teenagers experiencing some form of acne 1 The prescription acne market saw about $2.3 billion in sales in 2019 2 There have been few novel topical treatments approved in recent years, with most new products being reformulations of old compounds Psoriasis Psoriasis affects about 2% of the world's population with about 80% being affected by plaque-type psoriasis 3 Over 75% of patients with plaque-type psoriasis have mild disease, but they have few treatment options as most effective products are limited to moderate-to-severe disease 3 Rosacea There are about 16 million patients with rosacea in the US and topical prescription products did about $374 million in sales in 2019 2 Like acne, there have been few novel topical treatment options approved in recent years and most have unwanted side effects Aesthetics The American Society of Plastic Surgeons estimates that over 15.4 million cosmetic procedures were performed in 2016 of which about 7 million used botulinum toxin 5 There has been a growing demand for aesthetic treatments, including from male patients Addressable Markets 1 – Guideline for Care and Management of Acne 2 – IQVIA, Inc. Top Line Market and Sales Analysis from years 2015-2020 3 – Guideline for Care and Management of Psoriasis 4 – Markets and Markets Medical Aesthetics Market Report – Medical Aesthetic Market Product, End User, Global Forecast to 2025; 2020 5 – Global trends of Botulinum Toxin

Complex freshwater sponge, Spongilla lacustris or Spongilla, harvested based on proprietary environmental conditions resulting in unique characteristics that are optimized for clinical applications Possesses multiple complementary chemical and mechanical properties to potentially enhance pharmaceutical treatment effect If approved, could be used as a standalone topical product or in a combination with macro molecules to enable intradermal delivery of drugs that typically must be injected Unique Natural Platform Technology
Chemical Components: Contains multiple chemical compounds that have demonstrated in-vitro: Anti-inflammatory activity: Reduction of C. acnes stimulated IL-8 production in NHEK inhibits IL-17A and IL-17F expression in human cell lines Anti-microbial activity against C. acnes Effects on sebum production (inhibits lipogenesis in sebocytes) Mechanical Component: Processed sponge contains a large number of uniquely sized siliceous spicules that exfoliate the dermal epithelium, thereby: Creating microchannels into the dermis for delivery of chemical compounds Opening closed comedones (blackheads) to increase oxygen Promoting collagen production (which accelerates the skin’s rejuvenation period) Dual Mechanisms of Action
Regulatory Planning to seek new drug exclusivity – 5 years No clear regulatory pathway for abbreviated NDAs (New Drug Applications) for approved botanical NDAs Commercialization of DMT310 Option to seek Rx-to-OTC switch after 5 years, due to safety and market for DMT310 resulting in substantially larger potential sales opportunity Patent Protection DMT310 patent application pending: 1 application pending DMT400 patent applications pending: DMT410 + botulinum toxin (8 applications pending) DMT420 + select biologics (1 application pending) DMT430 + dermal fillers (1 application pending) Raw Material Supply Signed an Exclusive Supply Agreement in February 2020 with one of the larger harvesters and suppliers of Spongilla raw material from a location with known commercial quantities available Supplier is a well-established company with over 18 years experience collecting and processing large quantities of raw material per year Supplier uses propriety harvesting information and licensed harvesters with boats and harvesting equipment Chemistry, Manufacturing, Controls Proprietary potency release assay Exclusivities and Intellectual Property Strategies
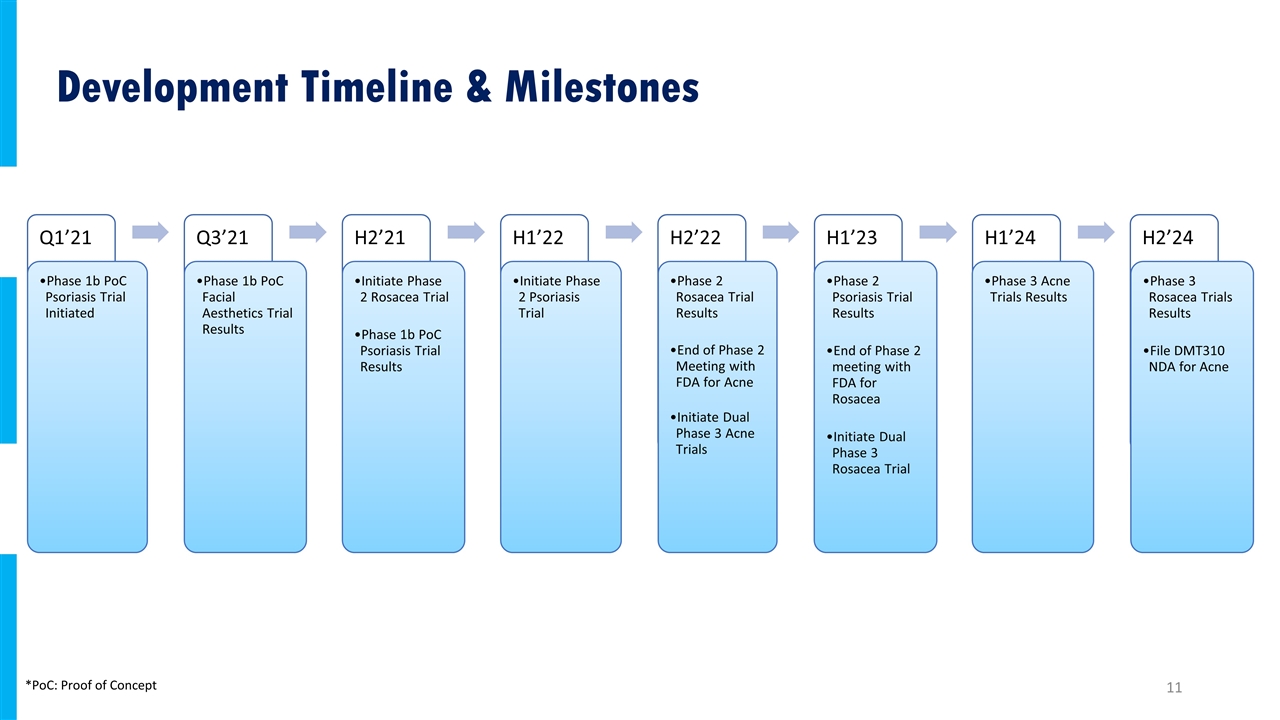
Development Timeline & Milestones *PoC: Proof of Concept Q1’21 Q3’21 H2’21 H1’22 H2’22 H1’23 H1’24 H2’24 Phase 1b PoC Psoriasis Trial Initiated Phase 1b PoC Facial Aesthetics Trial Results Initiate Phase 2 Rosacea Trial Phase 1b PoC Psoriasis Trial Results Initiate Phase 2 Psoriasis Trial Phase 2 Rosacea Trial Results End of Phase 2 Meeting with FDA for Acne Initiate Dual Phase 3 Acne Trials Phase 2 Psoriasis Trial Results Initiate Dual Phase 3 Rosacea Trial Phase 3 Acne Trials Results Phase 3 Rosacea Trials Results File DMT310 NDA for Acne End of Phase 2 meeting with FDA for Rosacea

DMT310: Once-weekly topical treatment
Frequency of treatments Current topical treatments are onerous and require 1-2 applications per day, resulting in poor compliance and early discontinuation by patients We believe DMT310 (once-weekly application) could optimize compliance Time to treatment effect Current topical treatments may take 6-8 weeks before a patient perceives a treatment effect DMT310 demonstrated statistically significant reductions in inflammatory and non-inflammatory lesions after one month with only 4 treatments, and continued reduction through week 12 versus placebo Side effects/Tolerability For many patients using available products, side effects and tolerability issues, such as burning, stinging, and peeling, occur well before a treatment effect, resulting in poor overall compliance We believe DMT310’s side effect/tolerability profile coupled with its comparatively fast onset of action could result in better compliance, leading to better outcomes and greater patient satisfaction DMT310 Addresses Unmet Needs in Acne, Psoriasis and Rosacea
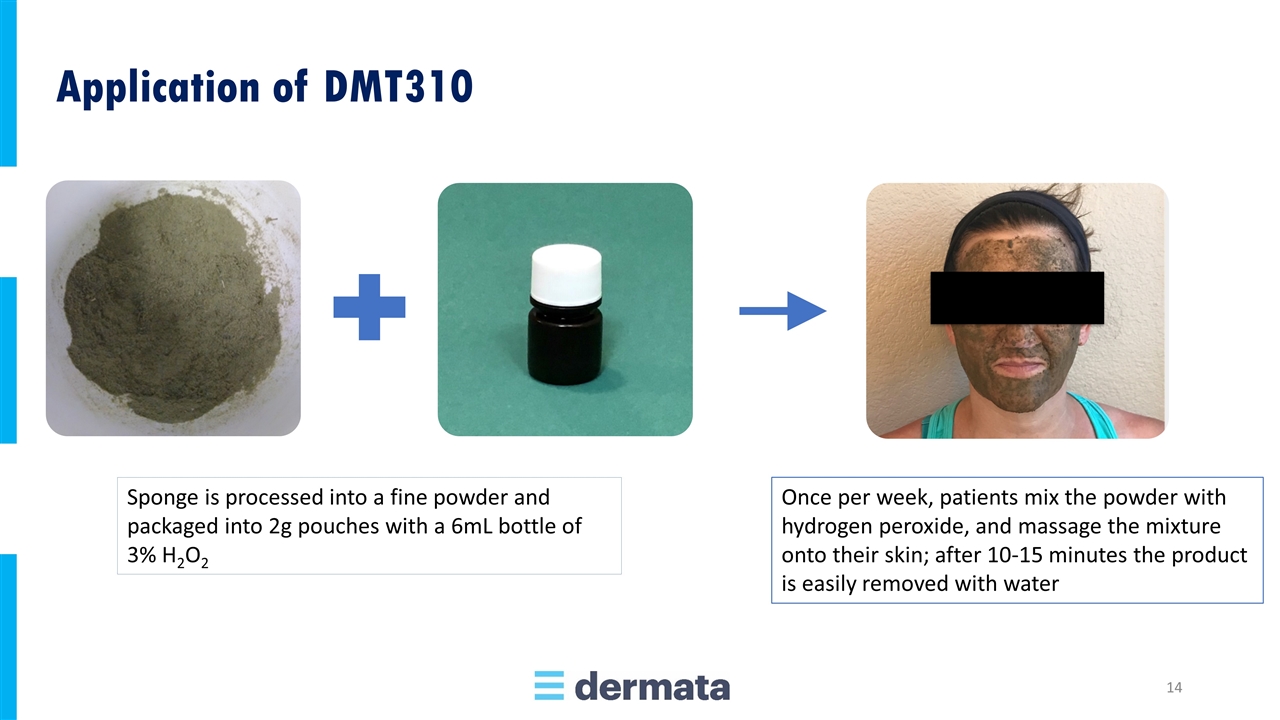
Once per week, patients mix the powder with hydrogen peroxide, and massage the mixture onto their skin; after 10-15 minutes the product is easily removed with water Sponge is processed into a fine powder and packaged into 2g pouches with a 6mL bottle of 3% H2O2 Application of DMT310
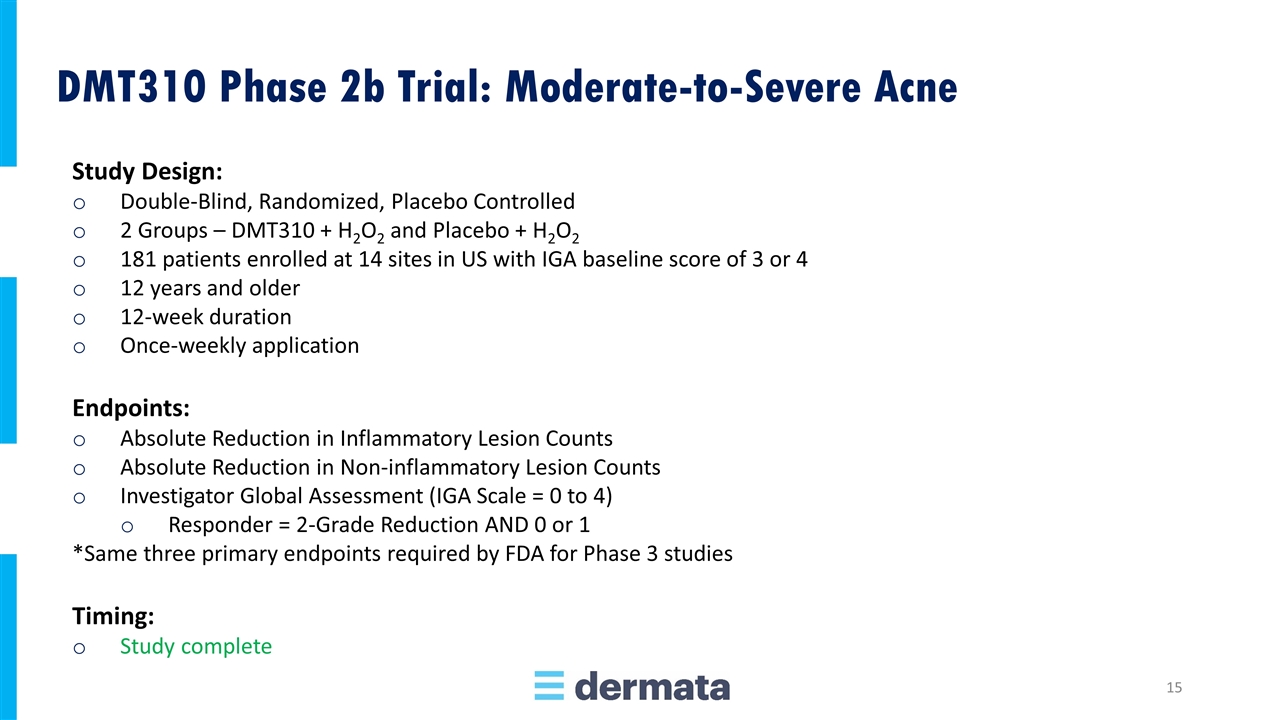
Study Design: Double-Blind, Randomized, Placebo Controlled 2 Groups – DMT310 + H2O2 and Placebo + H2O2 181 patients enrolled at 14 sites in US with IGA baseline score of 3 or 4 12 years and older 12-week duration Once-weekly application Endpoints: Absolute Reduction in Inflammatory Lesion Counts Absolute Reduction in Non-inflammatory Lesion Counts Investigator Global Assessment (IGA Scale = 0 to 4) Responder = 2-Grade Reduction AND 0 or 1 *Same three primary endpoints required by FDA for Phase 3 studies Timing: Study complete DMT310 Phase 2b Trial: Moderate-to-Severe Acne
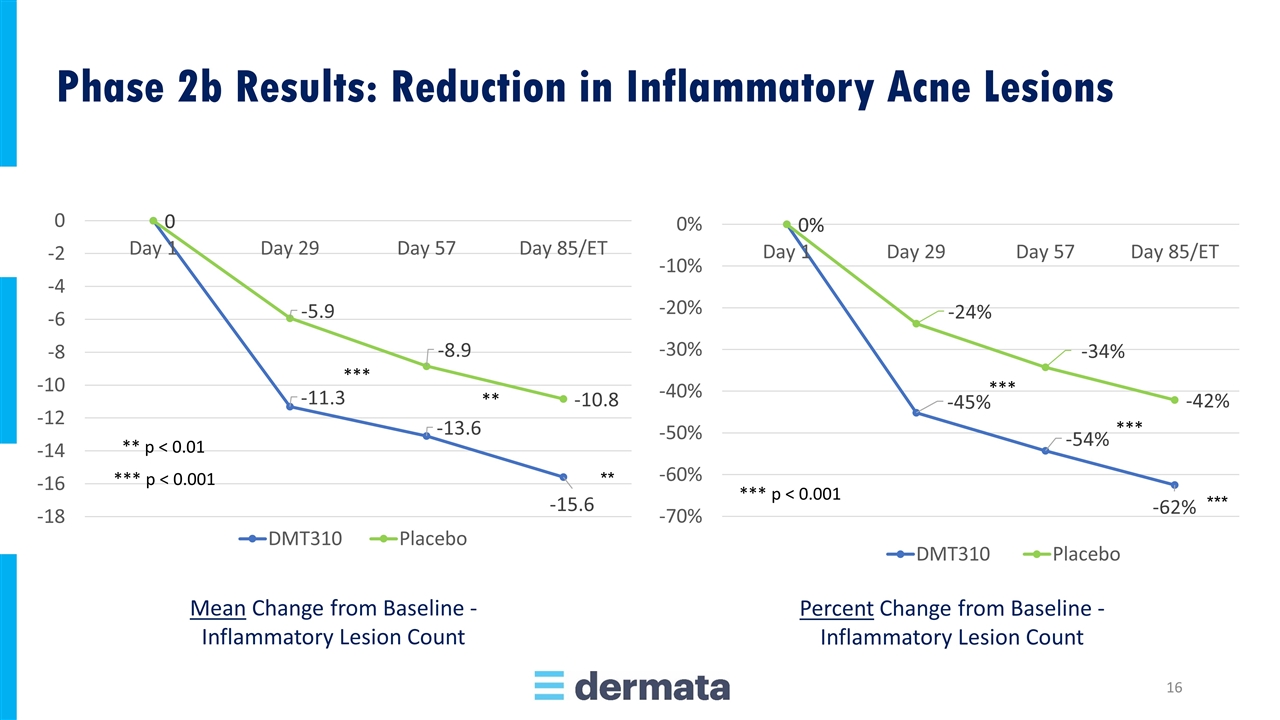
Mean Change from Baseline - Inflammatory Lesion Count Percent Change from Baseline - Inflammatory Lesion Count Phase 2b Results: Reduction in Inflammatory Acne Lesions
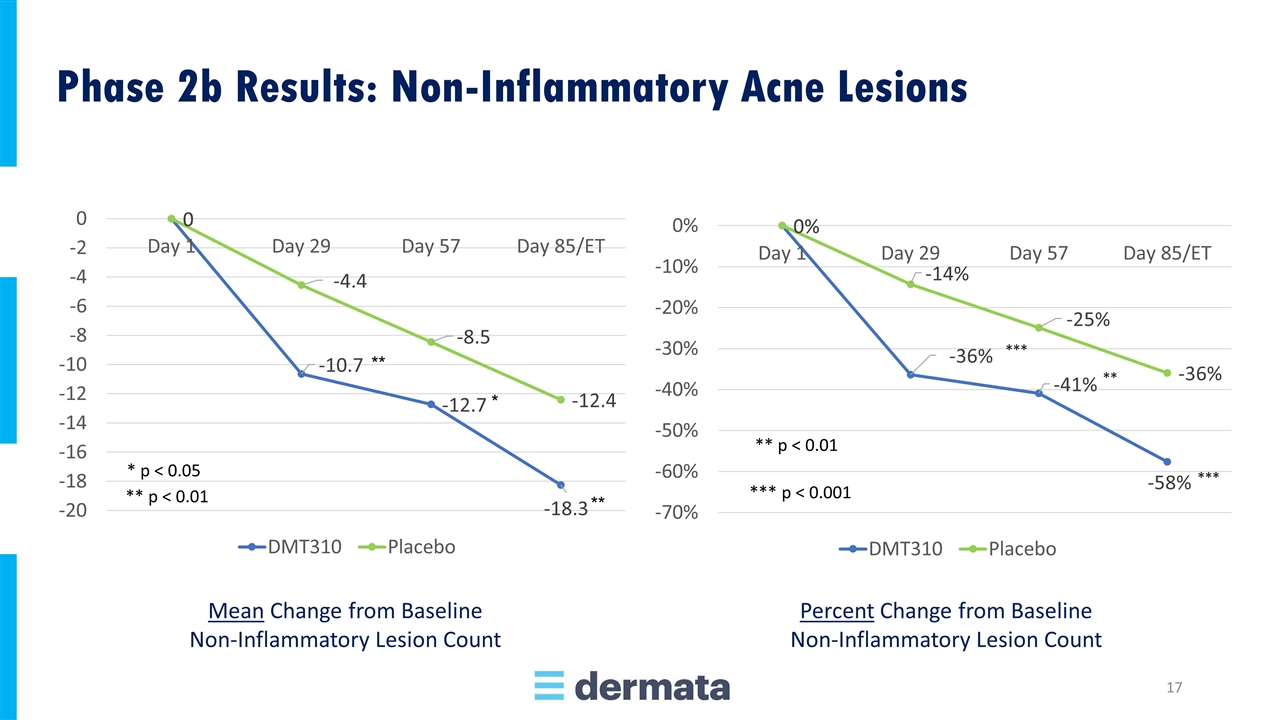
Mean Change from Baseline Non-Inflammatory Lesion Count Percent Change from Baseline Non-Inflammatory Lesion Count Phase 2b Results: Non-Inflammatory Acne Lesions
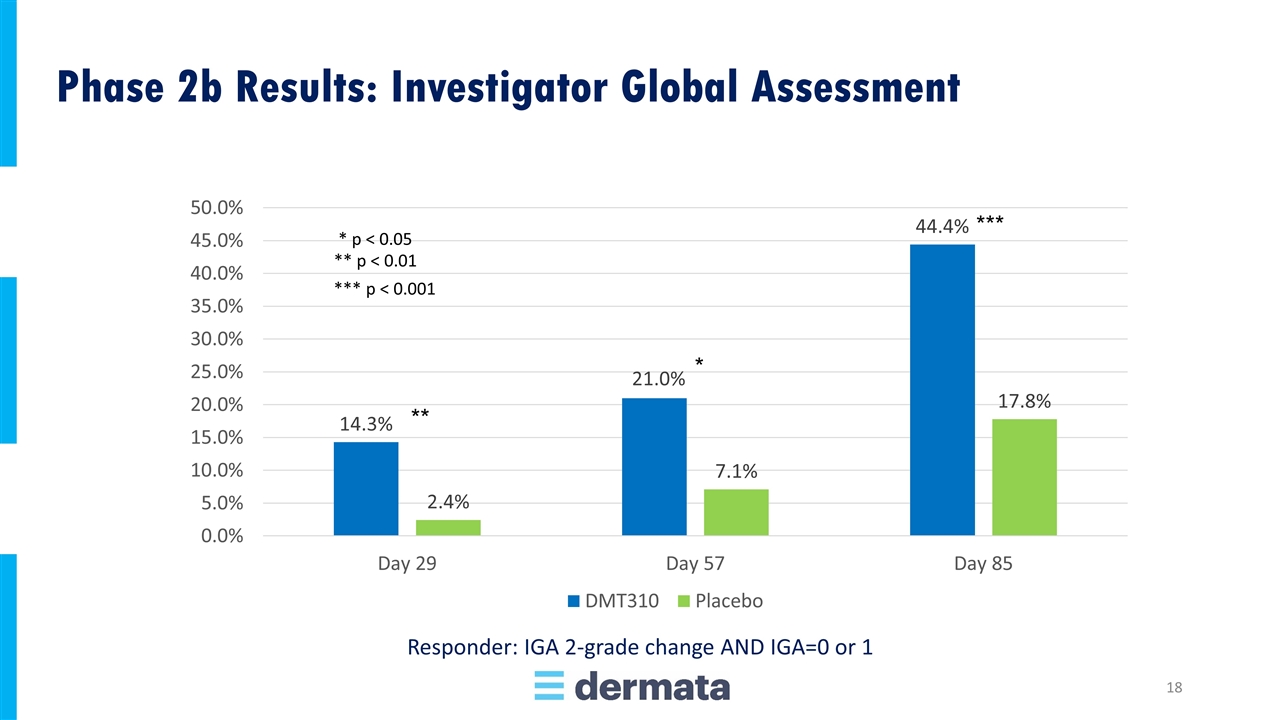
* p < 0.05 ** p < 0.01 Responder: IGA 2-grade change AND IGA=0 or 1 Phase 2b Results: Investigator Global Assessment
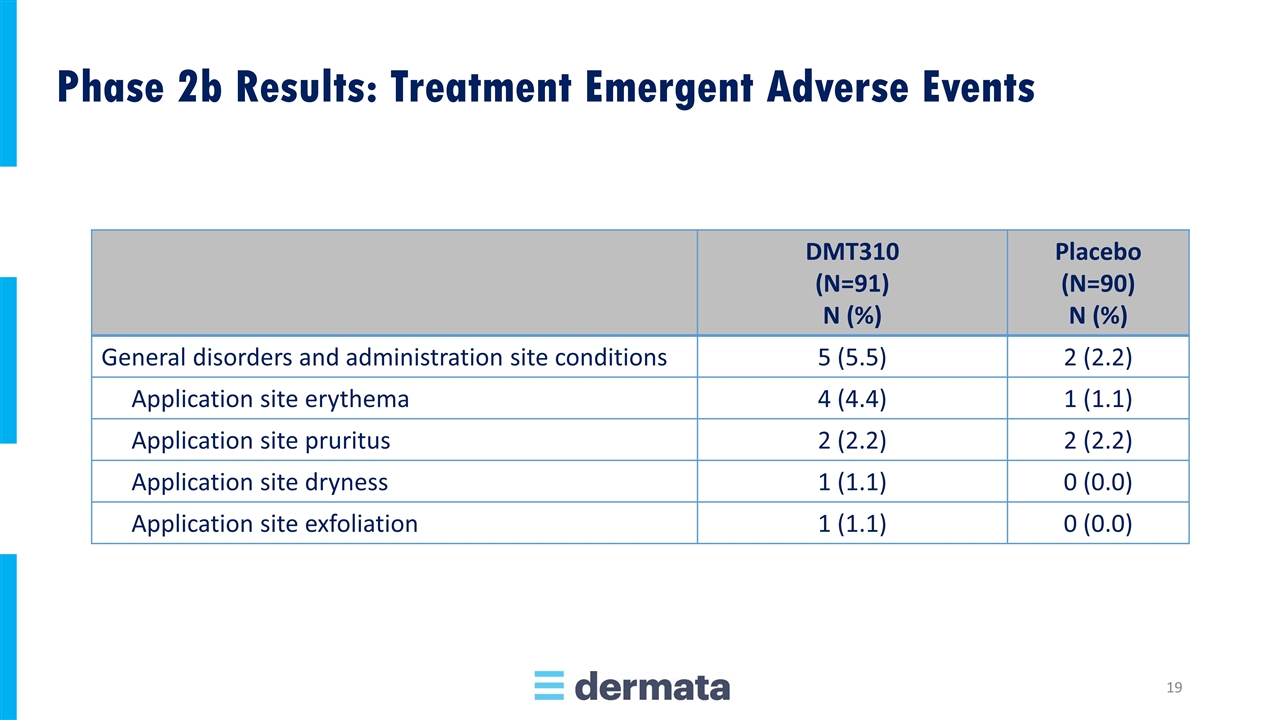
DMT310 (N=91) N (%) Placebo (N=90) N (%) General disorders and administration site conditions 5 (5.5) 2 (2.2) Application site erythema 4 (4.4) 1 (1.1) Application site pruritus 2 (2.2) 2 (2.2) Application site dryness 1 (1.1) 0 (0.0) Application site exfoliation 1 (1.1) 0 (0.0) Phase 2b Results: Treatment Emergent Adverse Events
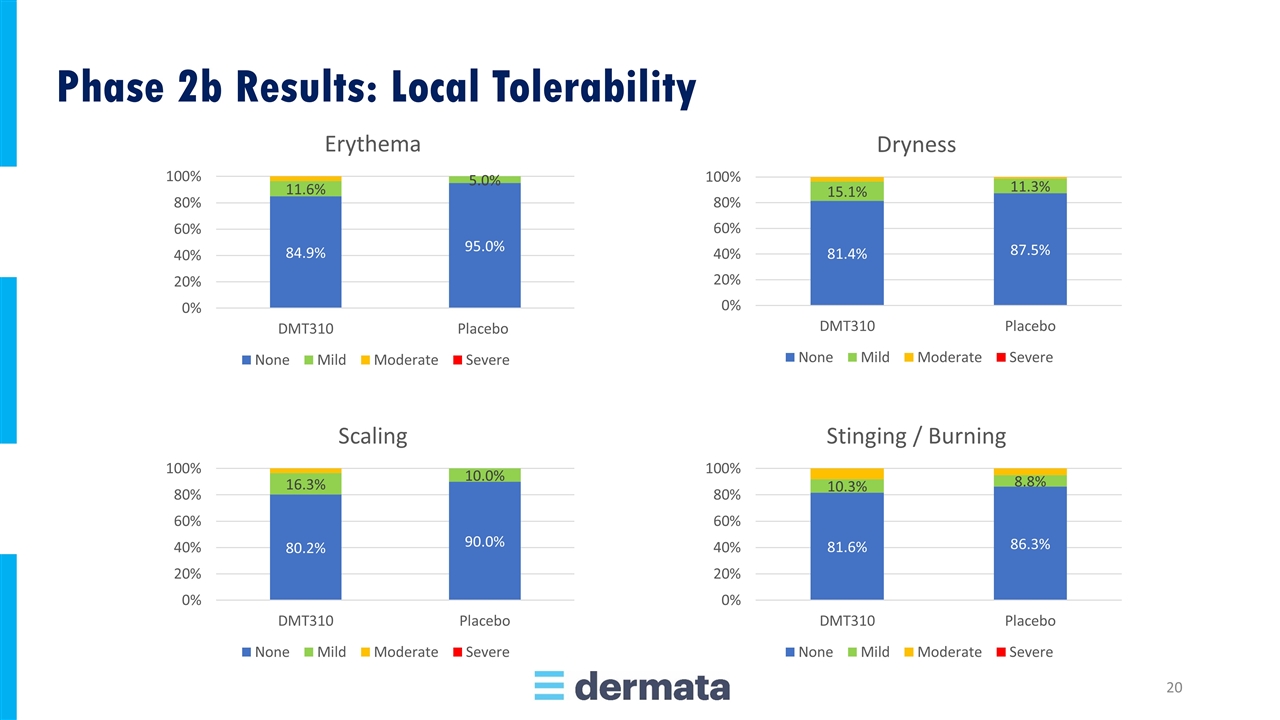
Phase 2b Results: Local Tolerability
Study Design: Phase 1b Proof-of-Concept, open label study DMT310 + H2O2 30 patients enrolled at 3 sites in US with psoriatic plaque covering 2% to 30% of BSA 18 years and older 12-week duration Once-weekly application Endpoints: 6-point Investigator’s Physician’s Global Assessment (PGA) at the target lesion site at Week 12 6-point Investigator’s Psoriasis Area Severity Index (PASI) scale at the target lesion site at the Week 12 Pruritus Visual Analog Scale (VAS) assessment of the target lesion Timing: First patient, first visit – March 2021 Last patient, last visit – H2 2021 Top-line results – H2 2021 DMT310 Phase 1b Trial: Mild-to-Moderate Psoriasis (Ongoing)
Study Design: Double-Blind, Randomized, Placebo Controlled 2 Groups – DMT310 + H2O2 and Placebo + H2O2 180 patients enrolled at 24 sites in US with IGA baseline score of 3 or 4 18 years and older 12-week duration Once-weekly application Endpoints: Absolute Reduction in Inflammatory Lesion Counts Investigator Global Assessment (IGA Scale = 0 to 4) Responder = 2-Grade Reduction AND 0 or 1 Timing: First patient, first visit – H2 2021 Last patient, last visit – H2 2022 Top-line results – H2 2022 DMT310 Phase 2 Trial: Moderate-to-Severe Rosacea (Planned)

DMT410: enabling topical application of botulinum toxin
*Spicules average about 200 µm in length, 10-15 µm in diameter Adapted from Zheng et al Biomater. Sci. 2019; 7 (4): 1299-1310 Botulinum toxin (>100 kD) Spicule creates microchannel for topical application of toxin DMT410 is a combination treatment regimen that uses sponge technology to allow for needle-free topical application of botulinum toxin, which customarily must be injected Spicule* DMT410’s Combination Regimen for Botulinum Toxin
Sponge mixture is massaged into the treatment area to enhance spicule penetration and create microchannels After 10-15 minutes the sponge mixture is removed Then botulinum toxin is expressed from a syringe in precise amounts onto the skin and massaged into the treatment area to take advantage of the newly created microchannels Application of DMT410
Molecule Size Botulinum toxin molecules are between 400-900 kDa and are currently injected for clinical efficacy We believe DMT410 creates microchannels in the skin that are large enough to allow topical penetration of botulinum toxin into the dermis for targeted treatment Increased Coverage Currently botulinum toxin is injected into the muscle or intradermally, limiting the spread of the toxin to the area around the injection We believe that with the creation of a significant number of microchannels, the topical application of toxin can more easily deliver toxin to a larger surface area Additional Uses Certain aesthetic conditions, fine lines, pore size, luminosity, brightness, etc., that botulinum toxin has shown improvement, but difficulty targeting treatment with injections limits its commercial opportunity We believe DMT410’s topical delivery allows the toxin to reach the layer of skin necessary for treatment of these aesthetic conditions No Injections Necessary Current treatments with botulinum toxin, like hyperhidrosis, require 10-20 injections in each axilla that can be painful for patients DMT410’s topical application had acceptable tolerability in our recent hyperhidrosis clinical trial DMT410 Increases Potential Benefits of Botulinum Toxin vs Injections
Study Design: Open-label, 2-arm study 2 Groups – DMT410 + H2O2 and DMT410 + H2O 10 patients enrolled at 1 site in US At least 18 years of age DMT410: One application of sponge powder, followed by one topical application of BOTOX® 4-week duration Endpoints: Percent of patients with ≥50% reduction in gravimetrically measured sweat production from baseline Percent of patients with gravimetric sweat production ≤ 50mg Percent change in gravimetric sweat production Timing: Study complete DMT410 Phase 1b Trial: Moderate-to-Severe Axillary Hyperhidrosis
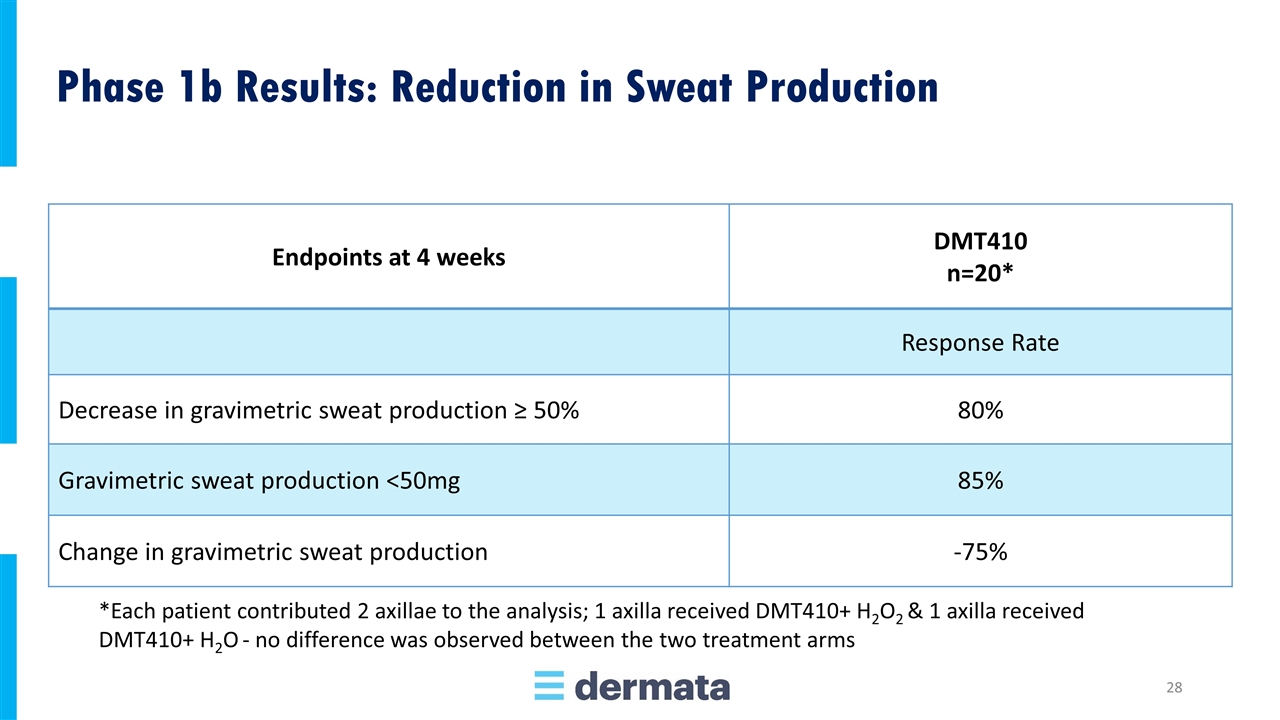
Endpoints at 4 weeks DMT410 n=20* Response Rate Decrease in gravimetric sweat production ≥ 50% 80% Gravimetric sweat production <50mg 85% Change in gravimetric sweat production -75% *Each patient contributed 2 axillae to the analysis; 1 axilla received DMT410+ H2O2 & 1 axilla received DMT410+ H2O - no difference was observed between the two treatment arms Phase 1b Results: Reduction in Sweat Production

Study Design: Open-label, 1-arm study 10 patients enrolled at 1 site in US One application of sponge mixture, followed by 1 topical application of BOTOX® At least 18 years of age Patients will be assessed at 4 weeks, 8 week, 12 weeks and 16 weeks post application Endpoints: Moderate to severe glabellar, lateral canthal, forehead lines, luminosity, brightness, pore size, sebum production, Global Aesthetic Improvement, laxity under the eye and fine lines under the eye Timing: First patient, first visit – November 2020 Last patient, last visit – H1 2021 Top-line results – Q3 2021 DMT410 Phase 1b Trial: Facial Aesthetics (Ongoing)
Corporate Highlights Unique, multi-use topical platform technology utilizing multiple mechanisms of actions Lead program with compelling Phase 2b clinical data for once-weekly topical treatment of acne Pipeline addressing large medical and aesthetic dermatology market opportunities Multiple clinical milestones anticipated in 2021 and 2022 Experienced management team and board DMT310 DMT410 Psoriasis: Phase 1b PoC Initiated – Q1’21 Potentially inhibits inflammatory cytokines IL-17A and IL-17F Anticipated once-weekly topical application Rosacea: Phase 2 Planned – H2’21 Similar inflammatory lesions to acne Reduces IL-17, which facilitates neutrophil recruitment Acne: Phase 3 Planned – H2’22 Potential anti-inflammatory effects Reduced lipogenesis of sebocytes in-vitro Hyperhidrosis: Phase 1b PoC Completed Topical delivery of botulinum toxin Reduction in sweat production Aesthetics: Phase 1b PoC Ongoing - Results Q3’21 Potentially broadens uses for botulinum toxin with topical applications Potentially decreases need for injections *PoC: Proof of Concept

Dermata Therapeutics Transforming topical treatment of the skin