
EXHIBIT 99.1

Dermata Therapeutics Provides Corporate Update and Reports Full Year 2022 Financial Results
- End of Phase 2 meeting with FDA for DMT310 for moderate-to-severe acne expected in 2Q 2023 –
- Initiation of DMT310 Phase 3 clinical trial program in moderate-to-severe acne patients expected in 2H 2023 -
- DMT410 partnering discussions ongoing -
SAN DIEGO, CA, February 21, 2023 – Dermata Therapeutics, Inc. (Nasdaq: DRMA; DRMAW) (“Dermata,” or the “Company”), a clinical-stage biotechnology company focusing on the treatment of medical and aesthetic skin conditions, today highlighted recent corporate progress, and reported financial results for the year ended December 31, 2022.
“We are very excited for what Dermata has planned for 2023 for both our DMT310 and DMT410 programs,” said Gerry Proehl, Dermata’s Chairman, President, and Chief Executive Officer. “We believe advancing the DMT310 acne program into Phase 3 will be a significant step for our company. I am confident that our team can continue forward progress and build upon the results we saw in our positive DMT310 Phase 2b acne study, in which almost 45% of patients achieved an investigator global assessment, or IGA, score of ‘clear’ or ‘almost clear’ at the end of the 12-week clinical study. We are also encouraged by the progress made to further develop DMT410 as a potential method to topically deliver botulinum toxin for hyperhidrosis and aesthetic skin conditions.”
Anticipated Upcoming Milestones
|
| · | Type C Meeting Request with FDA on DMT310 Chemistry, Manufacturing, and Controls (CMC) questions. Dermata has submitted a Type C meeting request to FDA with questions in order to receive feedback related to the CMC of DMT310 for Phase 3 and, if successful, a new drug application, or NDA, to FDA. The company expects to receive feedback from FDA in 1H 2023. |
|
|
|
|
|
| · | DMT310 End of Phase 2 Meeting with FDA. Dermata plans to request an end of Phase 2 meeting with FDA in 2Q 2023. Feedback from FDA will help guide Dermata’s design of its DMT310 Phase 3 clinical trial program in moderate-to-severe acne patients. |
|
|
|
|
|
| · | DMT310 Phase 3 Program in Moderate-to-Severe Acne. After receiving feedback from the end of Phase 2 meeting with FDA, the Company intends to initiate its DMT310 Phase 3 clinical trial acne program in 2H 2023. If the Phase 3 program is successful, the Company intends to submit an NDA to FDA seeking regulatory approval of DMT310 for moderate-to-severe acne. |
|
|
|
|
|
| · | DMT410 Partnership Discussions. Dermata continues to move forward with partnership discussions surrounding its DMT410 program for the topical delivery of botulinum toxin. |
| 1 |
Full Year 2022 Financial Results
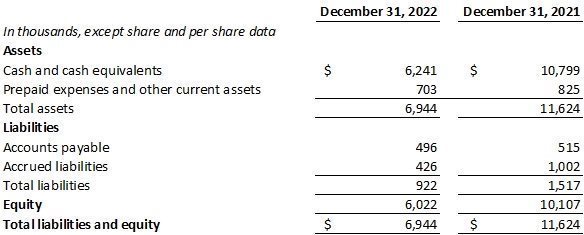
As of December 31, 2022, Dermata had $6.2 million in cash and cash equivalents, compared to $10.8 million as of December 31, 2021. Dermata expects its current cash resources are sufficient to fund operations into 3Q 2023.
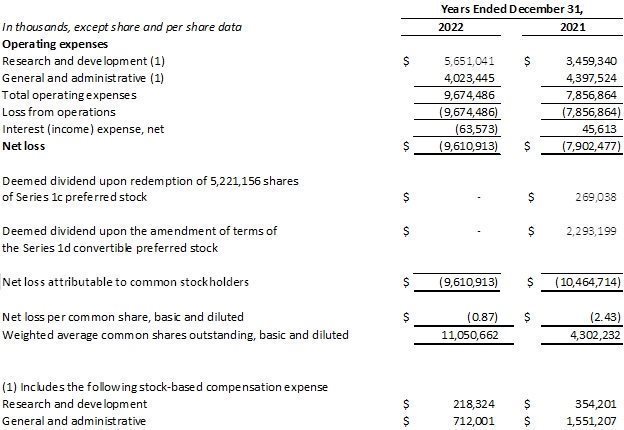
Research and development expenses were $5.7 million for the year ended December 31, 2022, compared to $3.5 million for the year ended December 31, 2021. The increase in research and development expenses was due to increased clinical, non-clinical, and CMC expenses for the DMT310 program. Stock-based compensation expense attributable to research and development totaled $0.2 million for the year ended December 31, 2022, compared to $0.4 million for the year ended December 31, 2021.
General and administrative expenses were $4.0 million for the year ended December 31, 2022, compared to $4.4 million for the year ended December 31, 2021. Stock-based compensation expense attributable to general and administrative totaled $0.7 million for the year ended December 31, 2022, compared to $1.6 million for the year ended December 31, 2021.
About Dermata Therapeutics
Dermata Therapeutics, Inc. is a clinical-stage biotechnology company focusing on the treatment of medical and aesthetic skin conditions. The Company’s lead product candidate, DMT310, is the Company’s first product candidate being developed from its Spongilla technology platform. DMT310 is a once-weekly topical product candidate derived from a naturally sourced freshwater sponge with multiple unique mechanisms of action. DMT310 has been studied for the treatment of acne, rosacea, and psoriasis. The Company’s second product candidate, DMT410, uses its Spongilla technology as a new method for topical intradermal delivery of botulinum toxin for the treatment of hyperhidrosis and multiple aesthetic skin conditions. Dermata is headquartered in San Diego, California. For more information, please visit http://www.dermatarx.com/.
Forward-Looking Statements
Statements in this press release that are not strictly historical in nature are forward-looking statements. These statements are based on the Company’s current beliefs and expectations and new risks may emerge from time to time. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions, and other factors including, but are not limited to, statements related to: expectations with regard to the timing of meetings and/or responses from submissions with regulatory bodies; the uncertainties inherent in clinical trials; expectations with regard to any potential partnership opportunities for any of the Company’s product candidates; the Company’s expectations with regard to current cash and cash equivalents and the amount of time it will fund operations; the success, cost, and timing of its product candidates DMT310 and DMT410 development activities and ongoing and planned clinical trials; and whether the results of any ongoing or planned clinical trials of DMT310 or DMT410 will lead to future product development. These statements are only predictions based on current information and expectations and involve a number of risks and uncertainties. Actual events or results may differ materially from those projected in any of such statements due to various factors, including the risks and uncertainties inherent in drug development, approval, and commercialization, and the fact that past results of clinical trials may not be indicative of future trial results. For a discussion of these and other factors, please refer to Dermata’s filings with the Securities and Exchange Commission. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. This caution is made under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All forward-looking statements are qualified in their entirety by this cautionary statement and Dermata undertakes no obligation to revise or update this press release to reflect events or circumstances after the date hereof, except as required by law.
| 2 |
DERMATA THERAPEUTICS, INC.
(Formerly Dermata Therapeutics, LLC)
Balance Sheets
| 3 |
DERMATA THERAPEUTICS, INC.
(Formerly Dermata Therapeutics, LLC)
Statements of Operations
Investors:
Sean Proehl
Senior Director, Legal and Business Development
info@dermatarx.com
| 4 |